
Regulatory agencies, including the US FDA, Japan Pharmaceutical and Medical Device Agency (PMDA), China Food and Drug Administration (CFDA), and the UK Medicines and Healthcare Products Regulatory Agency (MHRA), all use Phoenix WinNonlin to evaluate drug submissions. The NCA, PK/PD modeling tools, and statistical analysis capabilities in WinNonlin are used to support many different types of studies at Everest. It is the industry standard for non-compartmental analysis (NCA), pharmacokinetic/pharmacodynamic (PK/PD), and toxicokinetic (TK) modeling with a proven 30-year history. Everest uses Phoenix WinNonlin 8.1 to conduct pharmacokinetic (PK) and pharmacodynamic (PD) analysis, modelling and simulation, and non-compartmental analysis (NCA).

Phoenix WinNonlin is used by over 6,000 scientists at more than 1,500 establishments in 60 countries.
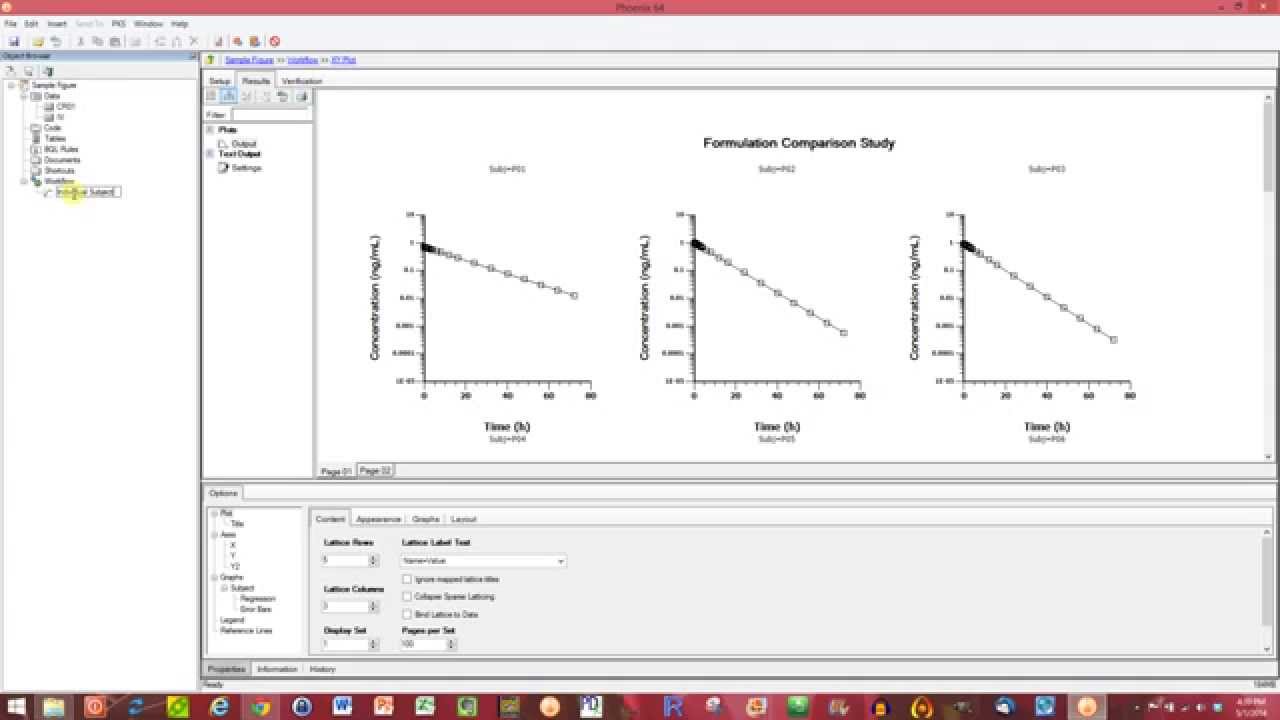

Phoenix WinNonlin™’s integrated tools for data processing, graphing & charting, report generation, and compliance create an efficient, all-in-one collaboration workbench. PK/PD and non-compartmental analyses can be time consuming, requiring detailed attention to every step from data preparation to report generation.


 0 kommentar(er)
0 kommentar(er)
